Introduction: Chimeric antigen receptor T (CAR-T) cell therapy has achieved remarkable success in hematological malignancies. However, CAR-T cell dysfunction due to exhaustion has been identified as a major roadblock to effective CAR-T cell therapy. Autophagy mediates T cell differentiation, survival, and memory by regulating the degradation of organelles and specific proteins. A recent study reported that inhibition of autophagy significantly enhanced the killing effect of CD19 CAR-T cells. Yet, the impact of autophagy inhibitors on CAR-T cell exhaustion and maintenance is still unclear. The study aims to systematically evaluate the role and mechanism of autophagy inhibitors on CAR-T cells.
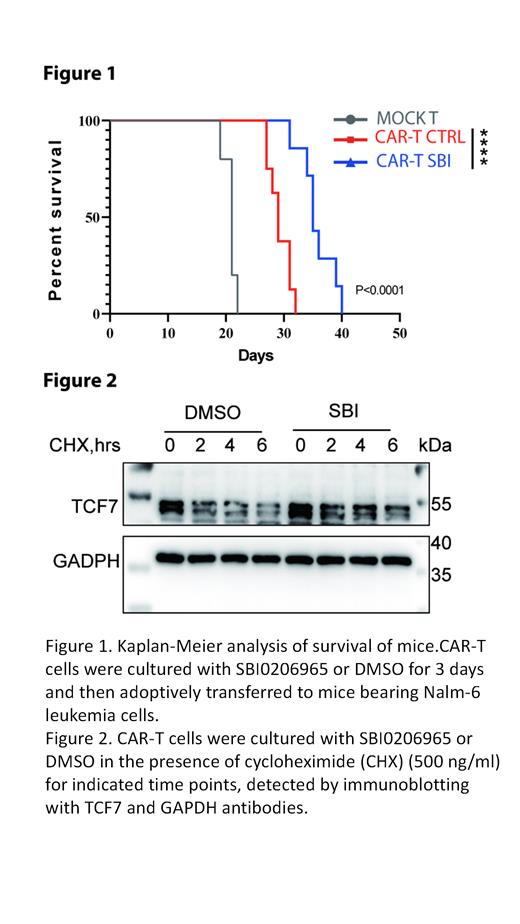
Methods and Results: To explore whether autophagy inhibitors could prevent CAR-T cell exhaustion triggered by antigen-independent CAR tonic signaling, we applied 3 autophagy inhibitors: SBI0206965, MRT67307, Compound C, respectively, to the culture medium of CD19.4-1BBz CAR-T cells. We found that all the 3 autophagy inhibitors could reduce CAR-T cell exhaustion and terminal differentiation. The results were replicable with GD2.28z CAR-T cells. Among the three inhibitors, SBI0206965 showed the most significant effect. Thus, we chose SBI0206965 for further research. CD19.4-1BBz CAR-T cells pretreated with SBI0206965 demonstrated enhanced killing capacity against Nalm-6 cells in vitro. Using the Nalm-6-bearing leukemia xenograft model, we found that compared to DMSO pretreated CD19.4-1BBz CAR-T cells, SBI0206965 pretreated CD19.4-1BBz CAR-T cells showed superior persistence and antitumor efficacy, exhibited a less exhausted and differentiated state, and further prolonged mice survival (Fig.1). To evaluate whether SBI0206965 could protect CAR-T cell exhaustion triggered by antigen stimulation, we cocultured CD19.4-1BBz CAR-T cells with Nalm-6 cells in the medium with or without SBI0206965. We demonstrated that the addition of SBI0206965 could effectively inhibit CAR-T cell exhaustion and terminal differentiation driven by antigen exposure. By performing proteomics, we identified TCF7 enriched in SBI0206965-treated cells. We validated the differences using Western blot analysis, which showed increased TCF7 levels in CAR-T cells treated with SBI0206965 for 24 hours. Thus we speculated that inhibition of autophagy might contribute to the accumulation of TCF7 and further improve CAR-T maintenance. To further verify whether TCF7 was degraded in an autophagy-dependent manner, we next determined TCF7 turnover rates in the presence of cycloheximide (CHX) in CAR-T cells with or without SBI0206965 treatment. CHX chase assay showed that SBI0206965 treatment could slower the TCF7 degradation rate(Fig.2).
Significance: Our research provides a strategy to optimize CAR-T antitumor efficacy and persistence via TCF7 accumulation by autophagy inhibition.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal